Differentiation Syndrome Pocket Card
Download the Differentiation Syndrome Pocket Card to carry with you and share with healthcare providers or emergency responders should you have signs and symptoms of differentiation syndrome.

147 adults with relapsed or refractory AML with an IDH1 mutation
Received REZLIDHIA treatment 150 mg twice a day by mouth
Until their healthcare provider discontinued treatment due to stem cell transplant, side effects, or worsening of their AML
The goal of AML treatment is to achieve remission—success with REZLIDHIA was measured by the number of people who went into remission.
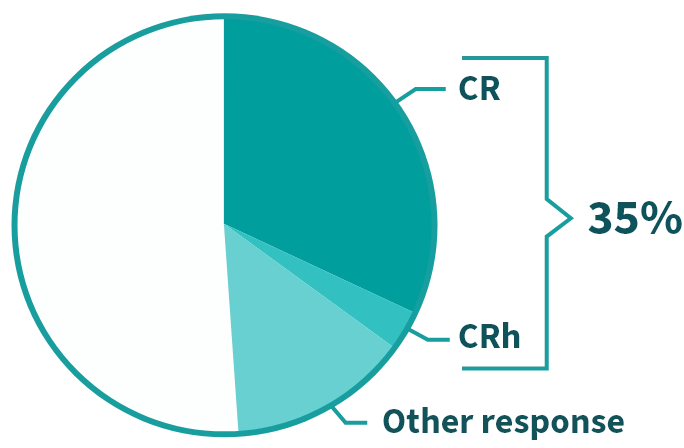
Complete remission (CR) means there were no signs of cancer and lab values returned to normal.
Complete remission with partial hematologic recovery (CRh) means there were no signs of cancer, but some lab values did not completely return to normal.
There are other types of responses to REZLIDHIA beyond CR or CRh. These include CR with incomplete recovery (CRi), partial response (PR), and morphologic leukemia–free state (MLFS). These “other responses” are also considered clinically meaningful.
35% of people taking REZLIDHIA achieved CR/CRh.
Half of people who achieved CR/CRh stayed in remission for over 26 months.

People who responded to REZLIDHIA did so as early as 1 month and up to 6 months. Half of people responded in the first 2 months.

34% of people who required transfusions at the start of the clinical trial became transfusion free for at least 8 weeks.

11% of people underwent a stem cell transplant following treatment with REZLIDHIA.
Everyone responds differently to treatment. During the REZLIDHIA trial, some side effects did occur, of which some were serious, resulting in dose adjustments or treatment discontinuation. It’s important to review the possible side effects with your healthcare provider before starting treatment.

Blood cells form through a process called differentiation. If this happens too quickly with AML treatment, differentiation syndrome can occur and may be life threatening or lead to death. Differentiation syndrome in adults with AML has occurred as early as 1 day and up to 18 months after starting REZLIDHIA.
Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome while taking REZLIDHIA:
If you develop differentiation syndrome, your healthcare provider may choose to monitor you in the hospital and will provide appropriate treatment.

Changes in liver function tests are common during treatment with REZLIDHIA and can be serious. Your healthcare provider will do blood tests to check your liver function before and during treatment with REZLIDHIA.
Tell your healthcare provider right away if you develop any of the following symptoms of liver problems during treatment with REZLIDHIA:

Tell your healthcare provider if you have any nausea, constipation, diarrhea, vomiting, stomach pain, or mouth sores.
Your healthcare provider will do blood tests before you start and during treatment with REZLIDHIA. Your healthcare provider may decrease, temporarily hold, or permanently stop your treatment with REZLIDHIA if you develop certain side effects. These are not all the possible side effects of REZLIDHIA.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is REZLIDHIA?
REZLIDHIA is a prescription medicine used to treat adults with acute myeloid leukemia (AML) with an isocitrate dehydrogenase-1 (IDH1) mutation when the disease has come back or has not improved after previous treatment(s).
Your healthcare provider will perform a test to make sure that REZLIDHIA is right for you.
It is not known if REZLIDHIA is safe and effective in children.
IMPORTANT SAFETY INFORMATION
REZLIDHIA may cause serious side effects including:
Differentiation Syndrome. Differentiation syndrome is a condition that affects your blood cells and may be life-threatening or lead to death. Differentiation syndrome in adults with acute myeloid leukemia (AML) has occurred as early as 1 day and up to 18 months after starting REZLIDHIA. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome during treatment with REZLIDHIA:
If you develop signs and symptoms of differentiation syndrome, your healthcare provider may treat you with a corticosteroid medicine or a medicine called hydroxyurea and may monitor you in the hospital.
Liver problems. Changes in liver function tests are common during treatment with REZLIDHIA and can be serious. Your healthcare provider will do blood tests to check your liver function before and during treatment with REZLIDHIA. Tell your healthcare provider right away if you develop any of the following symptoms of liver problems during treatment with REZLIDHIA:
The most common side effects of REZLIDHIA in adults with AML include:
Tell your healthcare provider if you have any nausea, constipation, diarrhea, vomiting, stomach pain or mouth sores.
Your healthcare provider will do blood tests before you start and during treatment with REZLIDHIA. Your healthcare provider may decrease, temporarily hold, or permanently stop your treatment with REZLIDHIA if you develop certain side effects. These are not all the possible side effects of REZLIDHIA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Before taking REZLIDHIA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Click here for Full Prescribing Information, including Boxed WARNING and Medication Guide.