
About AML & Rezlidhia
WHAT IS AML?
AML stands for acute myeloid leukemia, a type of blood cancer that originates in bone marrow, where immature cells, called myeloid cells, are born.

Myeloblast

Red blood cell
White blood cell
Platelet
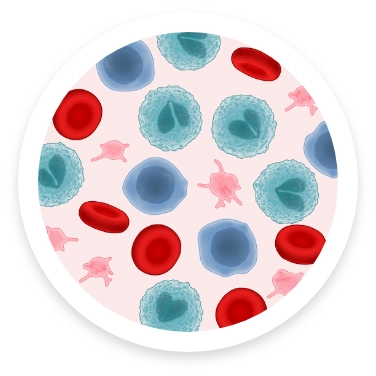
NORMAL BLOOD COUNT
Normally, immature myeloid cells develop into:
- Red blood cells that carry oxygen throughout the body
- White blood cells that fight infections
- Platelets that help the blood clot
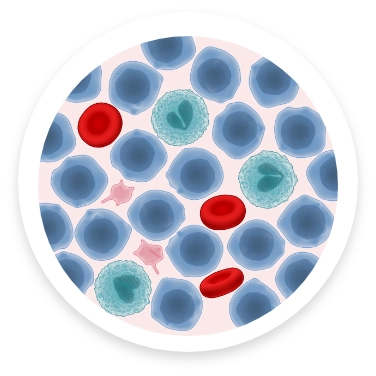
BLOOD COUNT WITH AML
With AML, immature myeloid cells cannot develop into normal blood cells and instead turn into cancer cells, called leukemic myeloblasts (immature blood cells), that grow and divide rapidly, crowding healthy cells and disrupting proper cell function. This results in symptoms associated with AML, such as:
- Weakness
- Fever
- Infection
- Paleness
- Bleeding
WHAT CAUSES AML?
In general, genetic mutations, or irregularities, can lead to AML. These mutations can often be detected through genetic testing.

WHAT IS AN IDH1 MUTATION?
An IDH1 gene normally makes a protein that helps break down fats for energy and protect cells. When an IDH1 gene is mutated, immature cells can’t develop properly. REZLIDHIA is a treatment designed specifically to target these IDH1 mutations.
WHAT IS RELAPSED OR REFRACTORY AML?
AML is typically categorized as newly diagnosed, relapsed, or refractory.
Relapsed AML means that your disease has come back after a period of improvement.
Refractory AML means that your disease hasn’t responded to treatment.
Usually, these types of AML are referred to together as relapsed or refractory AML.
What is REZLIDHIA?
REZLIDHIA is a targeted treatment for IDH1-mutated AML.
REZLIDHIA is a type of cancer medicine called an IDH1 inhibitor. It works differently than traditional chemotherapy.
By targeting the IDH1 gene mutation that prevents your blood cells from developing properly, REZLIDHIA helps the body return to normal blood cell development, function, and levels.

What is REZLIDHIA?
REZLIDHIA is a prescription medicine used to treat adults with acute myeloid leukemia (AML) with an isocitrate dehydrogenase-1 (IDH1) mutation when the disease has come back or has not improved after previous treatment(s).
Your healthcare provider will perform a test to make sure that REZLIDHIA is right for you.
It is not known if REZLIDHIA is safe and effective in children.
IMPORTANT SAFETY INFORMATION
REZLIDHIA may cause serious side effects including:
Differentiation Syndrome. Differentiation syndrome is a condition that affects your blood cells and may be life-threatening or lead to death. Differentiation syndrome in adults with acute myeloid leukemia (AML) has occurred as early as 1 day and up to 18 months after starting REZLIDHIA. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome during treatment with REZLIDHIA:
- fever
- cough
- trouble breathing
- decreased urination
- dizziness or lightheadedness
- weight gain
- swelling of your arms or legs
If you develop signs and symptoms of differentiation syndrome, your healthcare provider may treat you with a corticosteroid medicine or a medicine called hydroxyurea and may monitor you in the hospital.
Liver problems. Changes in liver function tests are common during treatment with REZLIDHIA and can be serious. Your healthcare provider will do blood tests to check your liver function before and during treatment with REZLIDHIA. Tell your healthcare provider right away if you develop any of the following symptoms of liver problems during treatment with REZLIDHIA:
- yellowing of your skin or the white part of your eyes (jaundice)
- dark “tea-colored” urine
- loss of appetite
- pain on the upper right side of your stomach area
- feeling very tired or weak
The most common side effects of REZLIDHIA in adults with AML include:
- abnormal liver function tests
- nausea
- feeling tired or unwell
- joint pain
- constipation
- shortness of breath
- fever
- rash
- mouth sores
- diarrhea
- changes in certain blood tests
Tell your healthcare provider if you have any nausea, constipation, diarrhea, vomiting, stomach pain or mouth sores.
Your healthcare provider will do blood tests before you start and during treatment with REZLIDHIA. Your healthcare provider may decrease, temporarily hold, or permanently stop your treatment with REZLIDHIA if you develop certain side effects. These are not all the possible side effects of REZLIDHIA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Before taking REZLIDHIA, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems.
- are pregnant or plan to become pregnant. REZLIDHIA may harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if REZLIDHIA passes into your breast milk. Do not breastfeed during your treatment with REZLIDHIA and for 2 weeks after your last dose of REZLIDHIA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Click here for Full Prescribing Information, including Boxed WARNING and Medication Guide.